Working on CDMO for Macromolecular Target Drugs

We have production talents with rich GMP experiences, environment and equipment in line with GMP, and perfect GMP management system, which provides the guarantee of the GMP production for you.
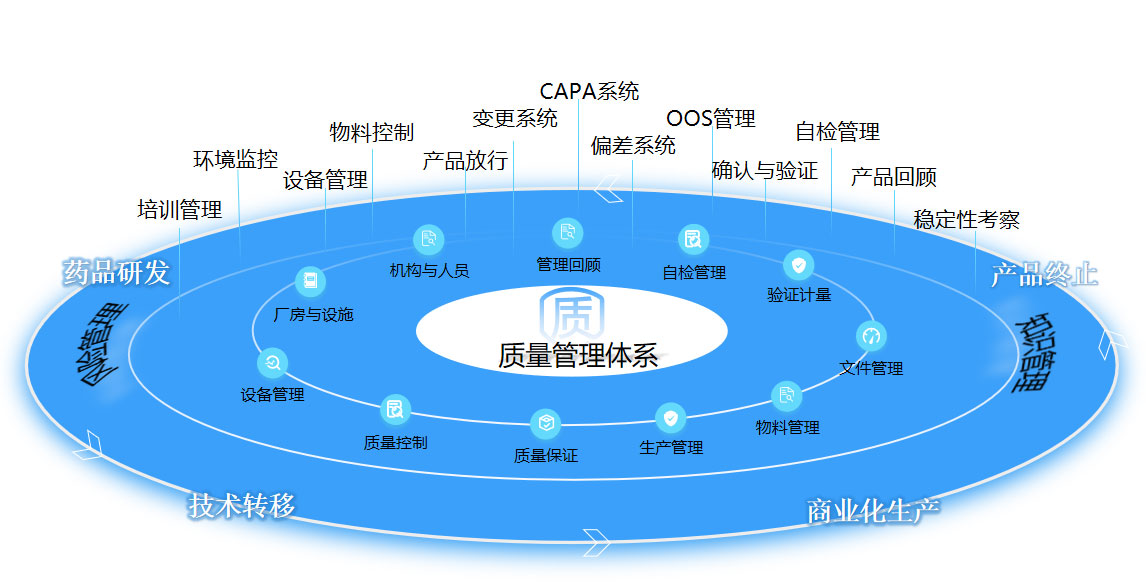
According to the requirements of ICH, EU, and China GMP, a quality management system covering the whole life cycle of products, such as early research and development, pre-clinical safety evaluation, clinical trials, and commercial production has been established to ensure that the drugs produced meet the intended use, registration requirements, and clients’ requirements.
For different stages of the product life cycle, we establish different quality system elements to guarantee the continuous improvement of the product and quality system. Change management system, deviation management system, correction and preventive system, monitoring system of product quality, review system of product quality, self-inspection management system, etc. are established to guarantee the operating and continuous improvement of products in different stages and quality systems. Knowledge and risk management are applied to put forward the product realization and let product R&D and production under control and continuous improvement. The company is dedicated to establishing a quality management system that complies with national standards to safeguard product quality.